To help increase the amount of helpful study content on this site, I’ve partnered with some medical students from East Tennessee State University! They are being mentored by one of my former ophthalmology residents, Dr. Brent Aebi. The posts are primarily authored by the medical student, and reviewed/edited by myself and Dr. Aebi.
This post and one-page coffee table book excerpt about Diabetic Retinopathy was written by DiAnna Rowe Presley, B.A.
Diabetic retinopathy (DR) is a serious complication of diabetes mellitus due to microvascular retinal ischemia. Among middle-aged adults, diabetic retinopathy is the leading cause of blindness around the world.
Pathophysiology
The common histopathologic hypothesis of diabetes-related microvascular disease is that prolonged hyperglycemia damages the capillary endothelium and results in pericyte loss. The basement membrane of these capillaries thickens and leads to capillary occlusion.
In the retina, these changes ultimately lead to capillary nonperfusion, resulting in a chronically ischemic state. The compromise of retinal capillary integrity causes perturbations in retinal vasculature structure, as well as may cause leakage of plasma and plasma products into the retinal tissue. Chronic retinal ischemia induces the production of vascular endothelial growth factor (VEGF), which promotes neovascularization membranes that can ultimately result in tractional retinal detachment.
Classification
The severity of the disease ranges from nonproliferative diabetic retinopathy to proliferative diabetic retinopathy. Diabetic macular edema (DME) may develop at any stage.
The classification of diabetic retinopathy is important for determining treatment interventions, assessing risk for vision loss, and scheduling follow-up exams.
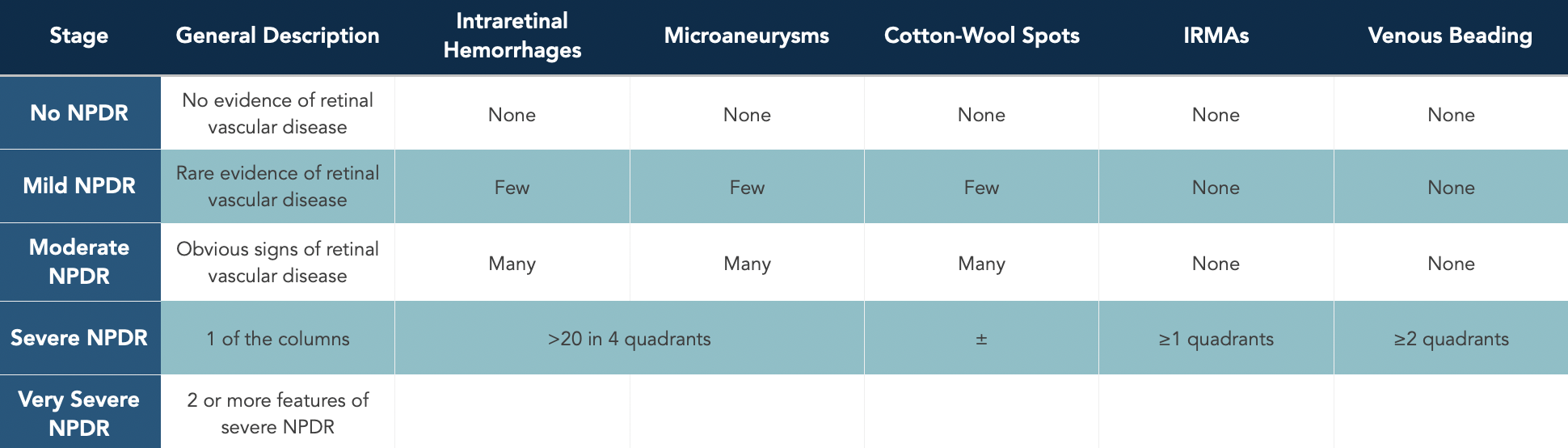
Nonproliferative Diabetic Retinopathy (NPDR)
Nonproliferative diabetic retinopathy (NPDR) is defined as vascular retinopathy related to diabetes mellitus that is confined to the retinal layers (i.e., does not extend beyond the internal limiting membrane).
The Early Treatment of Diabetic Retinopathy Study (ETDRS) defined the stages of NPDR. Severe NPDR (15%) and very severe NPDR (45%) were found to have a high risk for developing high-risk proliferative diabetic retinopathy (defined below) within a year.
The common retinal findings in NPDR include: intraretinal hemorrhages, microaneurysms, cotton-wool spots, intraretinal microvascular abnormalities (IRMAs), and dilation/beading of retinal veins.
Classification of nonproliferative diabetic retinopathy.
Proliferative Diabetic Retinopathy (PDR)
Proliferative diabetic retinopathy (PDR) develops after chronic retinal ischemia leads to aberrant angiogenesis in the retina, optic disc, or iris/angle neovascularization.
The Diabetic Retinopathy Study (DRS) classified PDR into low- and high-risk categories based on the extent of neovascularization.
Low-risk PDR is defined as neovascularization that does not meet high-risk characteristics.
High-risk PDR is defined as one of the following:
Neovascularization of more than 1/4-1/3 of the optic disc area, or
Neovascularization anywhere else associated with vitreous hemorrhage.
The primary treatment for both low and high-risk PDR is panretinal photocoagulation (PRP), in which a laser “kills” retinal tissue and induces scarring through coagulative necrosis. PRP reduced the risk of severe vision loss by at least 50% as compared to untreated eyes across severe NPDR, low-risk and high-risk PDR. The highest benefit of PRP was seen in eyes with high-risk PDR.
In some cases, intravitreal anti-VEGF injections is warranted, such as cases with vitreous hemorrhage where the peripheral retina cannot be visualized for PRP and injections help clear the hemorrhage. Patient ability for follow-up and reliability should be considered when deciding between anti-VEGF treatment and PRP therapy, as PRP offers long-lasting effects without need for frequent follow-ups (injections require monthly follow-up).
Diabetic Macular Edema
Macular edema can occur in both NPDR and PDR and reflects serum leakage from compromised retinal capillaries. Treatment is warranted to prevent vision loss and improve vision. First-line therapies are laser or anti-VEGF injections.
The ETDRS defined clinically-significant macular edema (CSME) in diabetes mellitus and established focal laser photocoagulation as an effective treatment. This definition, which was based on fundus visualization, has largely been supplanted with the refinement of optical coherence tomography (OCT) imaging. Today, “center-involving” and “non-center-involving” edema, as defined by thickening of the retinal layers based on OCT measurements, is a common classification.
Additionally, more recent studies, such as those from the Diabetic Retinopathy Clinical Research (DRCR) Network, have shown that anti-VEGF drugs (bevacizumab, ranibizumab, and aflibercept) are more effective than focal laser.
Follow-up for patients with central macular edema can be shortened to as frequently as every month. A graphic in the follow-up section below outlines follow-up in more detail.
Management of Diabetic Retinopathy
Many studies have contributed to determining the management of diabetic retinopathy. The details lie outside the scope of this article, but broadly speaking, treatment of DR falls into one of these two categories:
Promote vascular integrity: optimizing the microvascular disease risk factors of hyperglycemia, hypertension, and hyperlipidemia may allow retinal capillaries to heal to some degree, which in turn regresses some levels of diabetic retinopathy.
Reduce circulating intravitreal VEGF: because many of the vision-threatening sequela of DR are caused by VEGF, reducing intraocular VEGF essentially inactivates or stabilizes more advanced stages of diabetic retinopathy. This is achieved by either destroying ischemic retina through photocoagulation or by injecting anti-VEGF medications into the vitreous.
Systemic Disease Control
Strict glycemic control, blood pressure control and adequate lipid screening and treatment can stop progression. Earlier stages of the disease are managed by observation and metabolic optimization.
Laser Photocoagulation
Thermal laser photocoagulation has been a fixture in diabetic retinopathy treatment for over 40 years. Prior to the advent of anti-VEGF treatments, focal and grid laser photocoagulation were used in the treatment of diabetic macular edema. Panretinal photocoagulation (PRP) is still a commonly used treatment for proliferative diabetic retinopathy.
Because “dead” retina does not release VEGF, and VEGF is the molecule responsible for vision-threatening problems in PDR (neovascular membranes that lead to tractional retinal detachment, vascular permeability leading to diabetic macular edema), intentional and deliberate destruction of ischemic retina can dramatically reduce VEGF production and also promote increased oxygen diffusion into the retina through the choriocapillaris. Although this can be effective at halting progression, it comes at the cost of intentionally sacrificing parts of the peripheral retina that may still be fully functional. Extensive PRP can cause “tunnel” visual fields.
Anti-VEGF Injections
Anti-VEGF medications are now the first-line treatment of both diabetic macular edema and proliferative diabetic retinopathy. The indications for these agents lie outside the scope of this article.
Corticosteroids
Corticosteroids (topical, periocular, and intraocular) are used as second-line agents in the treatment of diabetic macular edema.
Vitrectomy
Vitrectomy is a treatment for some cases of proliferative diabetic retinopathy. Some of these indications include:
Anterior segment neovascularization with media opacity preventing PRP
Glaucoma
Hemolytic (erythroclastic or red blood cell-induced) glaucoma
Ghost cell glaucoma
Vitreous hemorrhage
Nonclearing
Substantial recurring despite maximum PRP
Subhyaloid hemorrhage involving macula (affecting vision)
Retinal detachment
Tractional retinal detachment involving or threatening the macula
Combined tractional and rhegmatogenous retinal detachment
Follow-Up
The follow-up schedule of patients with diabetic retinopathy can be summarized in a table [please see image below]. Follow-up depends on the severity, progression, and risk of vision loss.
Summary
In summary, diabetic retinopathy is a serious complication of a very common metabolic disease. It is imperative to first optimize preventative measures through glycemic and blood pressure control and move to intravitreal injections or laser intervention when vision is threatened. Overall, the management of diabetic retinopathy is complex and incorporates recommendations from landmark trials, patient’s treatment response, and severity of vision loss.
References
Basic and Clinical Science Course, Section 12: Retina and Vitreous. 2025-2026 Edition. San Francisco: American Academy of Ophthalmology, 2025. 99-130.
Diabetic Retinopathy Clinical Research Network Writing Committee. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469-480.
Download a copy of this coffee table book page excerpt for free!